Abstract
ABSTRACT
Introduction: Belumosudil (KD025) is a novel oral selective rho-associated coiled-coil-containing kinase-2 (ROCK2) inhibitor specifically designed for the treatment of cGVHD following an allogeneic hematopoietic cell transplant. It has been evaluated in the phase 2a dose-finding (KD025-208) and the pivotal phase 2 ROCKstar trials. Patients with cGVHD in the KD025-208 trial received belumosudil after failure of 1 to 3 prior systemic lines of therapy (LOTs), and those in the ROCKstar trial received belumosudil after failure of 2 to 5 prior systemic LOTs. The best overall response rate (95% CI) achieved, which was the primary end point, was 72% (64%-78%) across the 3 dosages (belumosudil 200 mg QD, 200 mg BID and 400 mg QD) that were studied across the 2 trials. This led to the recent FDA approval of the 200-mg QD dose for the treatment of adult and pediatric patients aged ≥12 years with cGVHD after failure of ≥2 prior systemic LOTs. FFS was a secondary end point in both trials.
FFS is an established composite indicator for treatment success in cGVHD, as it incorporates recurrent malignancy, nonrelapse mortality (NRM) and the absence of subsequent cGVHD therapy. Historic rates of FFS in a prior large observational study of patients with cGVHD after second-line systemic therapy were 56% at 6 months, 45% at 12 months and 31% at 24 months (Inamoto, Blood 2013). Given limited data from contemporaneous clinical trials on FFS and prognostic factors of treatment failure, we studied pooled FFS and its determinants from the KD025-208 and ROCKstar trials.
Methods: A total of 186 patients from the KD025-208 (n=54) and ROCKstar (n=132) trials treated with belumosudil 200 mg QD, 200 mg BID or 400 mg QD were analyzed . The median duration of belumosudil treatment was 9.9 months (range, 0.4-44.7 months). Prior therapies included tacrolimus (58%), sirolimus (46%), extracorporeal photopheresis (42%), mycophenolate mofetil (27%), ibrutinib (27%), ruxolitinib (21%) and cyclosporine (5%).
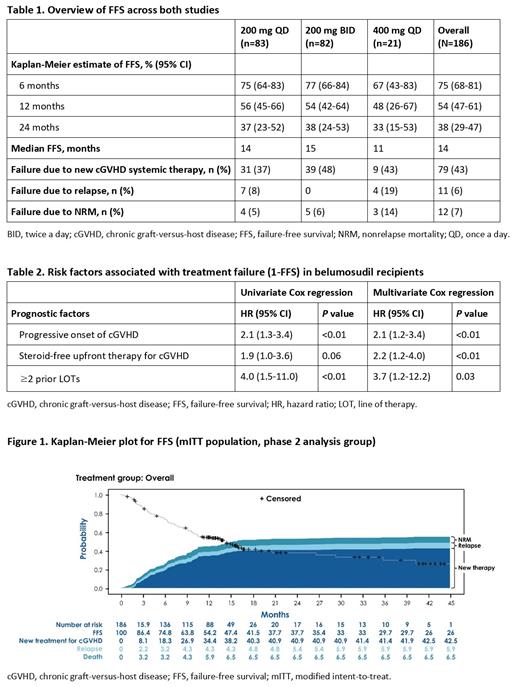
Results: At enrollment, 70% of patients had severe cGVHD according to NIH global score, 52% had ≥4 organs involved and 37% received >3 prior LOTs. Overall, belumosudil was well tolerated, with drug discontinuations occurring in 10% of patients due to possible drug-related adverse events. The median FFS was 14 months. The estimated overall FFS (95% CI) rates were 75% (68%-81%), 54% (47%-61%) and 38% (29%-47%) at 6, 12 and 24 months, respectively (Table 1 and Figure). Reasons for failure included recurrent malignancy (6%), NRM (7%) and the addition of a new systemic cGVHD therapy (43%). Factors associated with increased risk of treatment failure (1-FFS) in both univariate and multivariate analyses (Table 2) included progressive onset of cGVHD (multivariate hazard ratio [HR]=2.1 [1.2-3.4]), absence of glucocorticoids in upfront therapy for cGVHD (HR=2.2 [1.2-4.0]) and ≥2 prior LOTs (HR=3.7 [1.2-12.2]).
Conclusion: Treatment with belumosudil resulted in high FFS rates compared with historic benchmarks in cGVHD refractory to prior LOTs. Both NRM and relapse rates were low with the use of belumosudil. We identified distinct prognostic factors for FFS that can inform risk stratification and prognostication of patients being treated with belumosudil.
Lazaryan: Humanigen: Membership on an entity's Board of Directors or advisory committees; Avrobio: Membership on an entity's Board of Directors or advisory committees; Kadmon: Consultancy. Cutler: Mesoblast: Consultancy; Syndax: Consultancy; Omeros: Consultancy; Incyte: Consultancy; CareDx: Consultancy; Mallinckrodt: Consultancy; Pfizer: Consultancy; Kadmon: Consultancy; Editas: Consultancy; Cimeio: Consultancy; Deciphera: Consultancy; Jazz: Consultancy. Yang: Kadmon: Current Employment. Ieyoub: Kadmon: Current Employment. Eiznhamer: Kadmon: Current Employment. Blazar: BlueRock Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Carisma Therapeutics, Inc: Research Funding; Rheos Medicines: Research Funding; Tmunity Therapeutics: Other: Co-founder; Equilibre Pharmaceuticals Corp: Research Funding; Magenta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Lee: Amgen: Research Funding; Incyte: Research Funding; Novartis: Other: clinical trials, Research Funding; Pfizer: Research Funding; Kadmon: Research Funding; Takeda: Research Funding; Syndax: Research Funding; AstraZeneca: Research Funding; JANSSEN: Other; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees. Pavletic: Center for Cancer Research: Research Funding; National Cancer Institute: Research Funding; National Institutes of Health: Research Funding; Celgene: Research Funding; Actelion: Research Funding; Eli Lilly: Research Funding; Pharmacyclics: Research Funding; Kadmon: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal